Technical Sheets
This product contains the preservative sodium azide. The concentration percent of the sodium azide is ≤ .09%. Although this hazardous substance is a concentration below that required for the preparation of a Material Safety Data Sheet, we created a standard MSDS for your records.
Download Data SheetDownload MSDS
Reviews
Want to leave a review? Please click here to send us your review.
Worked Perfectly
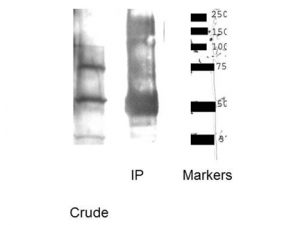
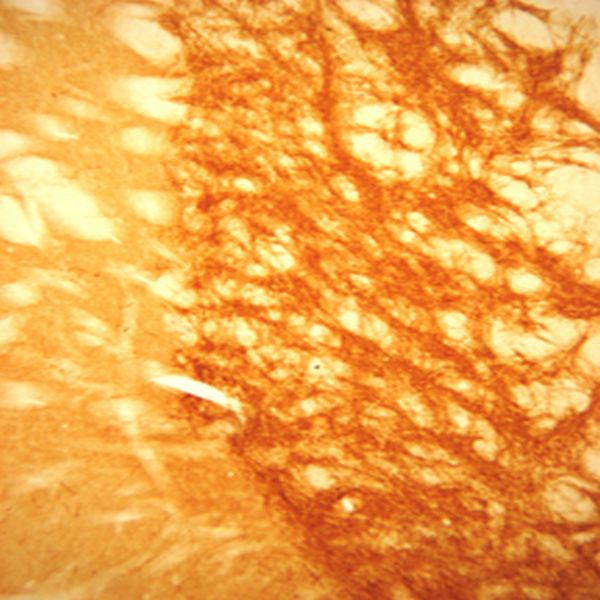
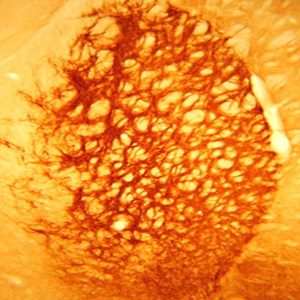
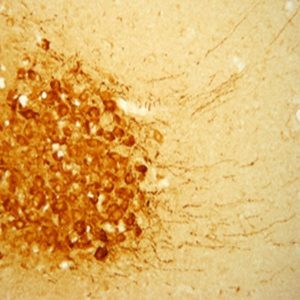
The SLC32A1 (ImmunoStar’s VIAAT Antibody # 20092) worked perfectly the first time on rat brain. We believe that the optimum antibody dilution is 1:20000. I included our imaging results from antibodies (see images above).
SLC32A1 Immunohistochemistry Staining Protocol:
Day 1:
1. Outline slides with ImmEdge Pen (waterproof marker)
a. It makes the outside hydrophobic => allows you to use less antibodies & prevents
b. Measure, using pipette, how much PBS soln comfortable covers outline slide
2. Wash sections 1×5 mins in PBS (shaken)
a. Use green box filled w/ PBS and submerse slides w/ the slide holder
b. When dumping off solution, place slides on a plastic surface b/c paper towels
3. Prepare blocking soln. Make on beaker with a stirrer (Triton is hard to dissolve). After preparation, keep excess on ice. Choose serum based on where the secondary antibody was raised (if raised in goat use NGS, if horse use NHS)
a. Use rectangular box w/ paper towel lightly soaked in PBS on the bottom
b. Only take out primary antibody aliquot when ready to use in step 7
c. ex. 7.5 mL Blocking Soln: 10% Serum + 3% BSA + 0.5% Triton + PBS spreading (~350 µL/slide) tend to have fibers that adhere to the slide surface
i. 750μ L Serum (Normal Horse Serum [NHS] or Normal Goat Serum [NGS])
ii. 1125μ L BSA (20% in PBS) [Bovine Serum]
iii. 37.5μ L Triton
iv. 5.59 mL PBS
4. Take out half of blocking soln and set aside for primary antibody soln
5. Apply original undiluted blocking soln (#6) for 90 mins
6. Take soln (#7) , mix w/ primary antibody
7. Apply antibody soln & incubate overnight @ +4 C
Day 2:
1. Wash sections 3×5 mins in PBS (shaken)
2. Apply secondary antibody solution for 60 mins. Prepare on ice. (Also make AB solution here it must rest for ≥ 30 mins not on ice)
a. ex. 5 mL soln: 1.5% BSA + PBS
i. 375μ L BSA (20% in PBS)
ii. 4625μ L PBS
iii. 25 μ L antirabbit antibody (1:200)
3. Wash sections 3×5 mins in PBS (shaken)
4. Apply Vectastain A & B w/ PBS for 60 mins (shaken @constant speed = 2)
a. ex. 1 drop A & 1 drop of B for every 2.5 mL of PBS (2 drops for 5 mL of PBS)
i. 50 μ L = 1 drop
ii. 20 μ L => 1 mL of PBS
b. Prepare soln at least 30 mins before use => allows for chemical rxn to occur
c. Binds to chromogen (DAB) resulting in reactional color change
5. Wash sections 3×5 mins in PBS (shaken)
6. Apply Filtered DAB in the fume hood for 5 mins, keep it on ice
7. Wash w/ PBS & then let the slides dry overnight
Day 3:
1. Place slides in glass slide holder
2. Immerse slides in old xylene soln under fume hood for 510 mins
3. Immerse slides in new xylene soln under fume hood for 510 mins
4. Take one coverslip out
5. Take out an individual slide from the holder in the xylene soln and put it near the coverslip
6. Coat with glue by dabbing on the samples quickly
7. Dip the coverslip in xylene & place it on the slide
8. Press evenly on the coverslip to spread the glue over the slide
a. Make sure the coverslip doesn’t hang off the sides of the slide
9. Quickly rinse slide in xylene to remove excess glue
10. Let it stand under fume hood to dry overnight
Submitted by:
Neal Rakesh
New York Medical College
MD Candidate