Description
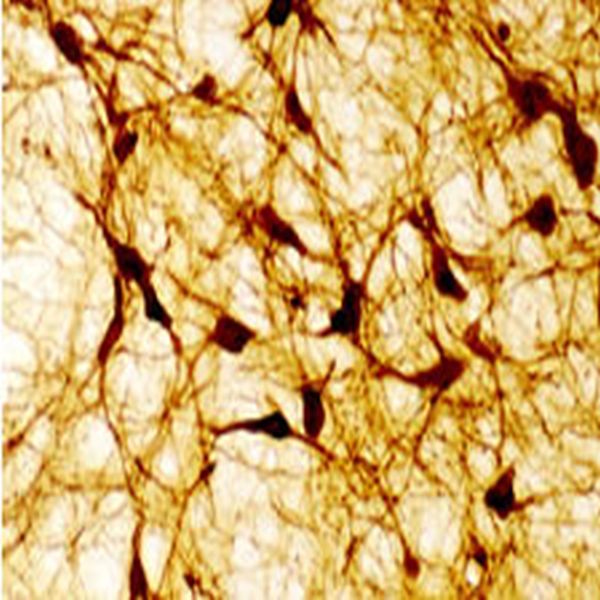
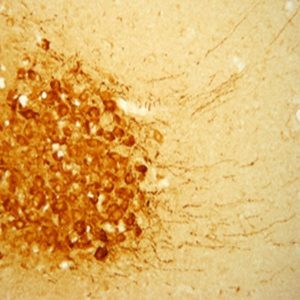
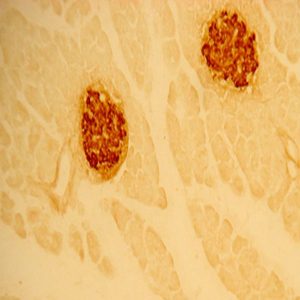
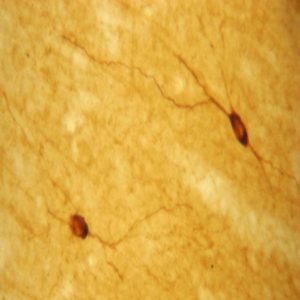
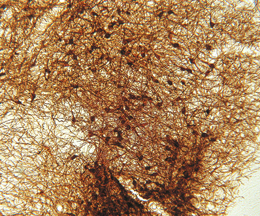
The ImmunoStar monoclonal Tyrosine Hydroxylase antiserum was quality control tested using standard immunohistochemical methods. The antiserum demonstrates strongly positive labeling of rat catecholamine neuron systems using indirect immunofluorescent and biotin/avidin-HRP techniques. Recommended primary dilution is 1/4000 – 1/8000 with the biotin/avidin-HRP technique. This antibody has been used successfully for IHC, ICC, FC, and IP. Western Blot: This antibody does not cross react with dihydropterdine reductase, dopamine-B-hydroxylase, phenylethanolamine-N-methyltransferase, phenylalanine hydroxylase or tryptophan hydroxylase using Western Blot methods. Click here for more Western Blot protocol information. The TH antibody identifies dopaminergic axons and nerve ending during early mouse, rat and human development. This is because tyrosine hydroxylase is expressed very early in the developing dopamine neuron phenotype. Photo Description: High magnification (above) and low magnification (below) IHC image of the rat midbrain staining for tyrosine hydroxylase. The tissue was fixed with 4% formaldehyde in phosphate buffer, before being removed and prepared for vibratome sectioning. Floating sections were incubated at RT in 10% rabbit serum in PBS, before standard IHC procedure. Primary antibody was incubated at 1:5000 for 48 hours, rabbit anti-mouse secondary was subsequently added for 1 hour after washing with PBS. Light microscopy staining was achieved with standard biotin-streptavidin/HRP procedure and DAB chromogen.
Host: Mouse
Quantity / Volume: 100 µL
State: Lyophilized Whole Serum
Reacts With: Alpaca (Llama), Amphibian, Anuran (Frog), Anuran (Urodele), Aplysia Californica (Sea Slug), Bee, Bird, Blowfly, Boar, Budgerigars, Canary, Cat, Chick, Chicken, Clam, Cockroach, Cod (Fish), Crab, Crayfish, Dog, Drosophila (Fly), Eel, Emu, Enegal Bichirs (Eel), Ewe, Ferret, Fish, Fly, Fox, Frog (Xenopus Laevis), Fruit Fly, Gastropod (Slug), Gastropoda, Gecko, Gerbil, Goldfish, Guinea Pig, Guppies, Hamster (Mesocricetus Auratus), Helisoma Duryi (Snail), Hen, Human, Iguana, Ilyanassa Obsoleta (Sea Snail), Lamprey, Leech, Lizard, Lobster, Locust, Mexican Axolotl (Salamander), Mollusca, Monkey (Macaca Fascicularis), Mouse, Newt, Opossum, Parrot, Periplaneta Americana (Cockroach), Phestilla Sibogae (Sea Coral), Phestilla Sibogae (Sea Slug), Phoronis Pallida (Phoronida), Pig, Pigeon, Possum, Prawn, Quail, Rabbit, Rat, Renilla Koellikeri (Sea Pansy), Rodent, Salamander, Salmon, Sea Fish, Sea Slug, Shark, Sheep, Short-Beaked Echidna, Shrew, Snail, Snake, Sparrow, Spisula (Clam), Squirrel Monkey, Starling, Stickleback, Tadpole, Trout, Turkey, Turtle, Urodele Amphibian, Water Buffalo, Worm, Yeast, Zebra Finch, Zebrafish
Availability: In Stock
Alternate Names: Tyrosine 3-monooxygenase (TH); TYH; Tyrosine 3-hydroxylase, anti-TH, anti-tyrosine hydroxylase
Gene Symbol: Th
Database Links:
Entrez Gene: 408930 Bee
Entrez Gene: 449568 Boar
Entrez Gene: 101090720 Cat
Entrez Gene: 395592 Chicken
Entrez Gene: 403444 Dog
Entrez Gene: 101682802 Ferret
Entrez Gene: 38746 Fly
Entrez Gene: 105299526 Fox
Entrez Gene: 100488900 Frog
Entrez Gene: 100734150 Guinea Pig
Entrez Gene: 100760127 Hamster
Entrez Gene: 7054 Human
Entrez Gene: 103238895 Monkey
Entrez Gene: 21823 Mouse
Entrez Gene: 102092212 Pigeon
Entrez Gene: 107314555 Quail
Entrez Gene: 100009362 Rabbit
Entrez Gene: 25085 Rat
Entrez Gene: 103186002 Shark
Entrez Gene: 101120619 Sheep
Entrez Gene: 102072945 Sparrow
Entrez Gene: 723977 Turkey
Entrez Gene: 101937054 Turtle
Entrez Gene: 100270767 Worm
Entrez Gene: 100219119 Zebra Finch
Entrez Gene: 30384 Zebrafish
Reviews
Want to leave a review? Please click here to send us your review.
Excellent Performance!
We use the Immunostar Tyrosine Hydroxylase antibody to label dopaminergic interneurons in the mouse olfactory bulb. It provides excellent signal with little background.
We typically use dilutions of 1:2000, but have gotten good results with dilutions up to 1:5000. The antibody is very reliable and works with fluorescent and chromogenic secondary antibodies. We highly recommend this antibody for its consistent performance and results.
Thomas Mast, PhD
Department of Biology
Eastern Michigan University
Ypsilanti, MI USA
Works great!
I use this mouse antibody for Tyrosine Hydroxylase for fluorescent immunohistochemistry (1:1000) and western blot (1:1000).
Tissue: cortex of mouse brain
IHC – 14 um coronally cryosectioned, perfused with Lana’s fix
WB – mouse brain cortex lysate, ECL imaging
This antibody works great in both of the techniques I use. Consistent staining and clear bands were shown.
Mo Kang
Integrated Program in Neuroscience
The Alan Edwards Centre for Research on Pain
McGill University
Wonderful Antibody!
This antibody produced excellent staining in bullfrog (Lithobates catesbeianus) brainstem using both Ni-DAB and IF. Staining was extremely clear with very little background at a concentration of 1:1000.
Tissue Preparation
Brainstem was removed from bullfrog and placed in 4% Paraformaldehyde overnight, then transferred to a 30% sucrose solution for at least 4 hours (until brain sinks). Brain was then placed in embedding media and flash frozen in hexane solution at -80 C. Brains were sliced to slides on a microtome to 20um. Slides were allowed to dry overnight prior to staining procedure.
For DAB Staining
Day 1
1. PBS 1X wash (3X5min)
2. H2O2 incubation 30min (room temperature)
3. PBS 1X wash (6X5min)
4. Blocking Buffer 1-2hr (PBS 1X+Normal serum+0.3% Triton 100X)
5. Primary antibody incubation overnight (PBS 1X+Normal serum+ 0.3% Triton 100X, Room Temperature)
Day 2
1. PBS 1X wash (5X5min)
2. Biotinylated secondary antibody incubation 1-2hr (PBS 1X+Normal Serum, Room Temperature)
3. PBS 1X wash (4X10min)
4. Incubate in ABC solution 1hr (Room Temp)
5. PBS 1X wash (6X5min)
6. Sodium Acetate wash (3X5min)
7. Ni-Sulfate DAB incubation 8min
8. 1-2 min H2O2 on Ni-DAB
9. Sodium Acetate wash (3X5min)
10. PBS 1X wash (3X5min)
This protocol worked great for TH staining in bullfrog and has been used for 2 years from frozen aliquots with great results.
Mitchell Reed, PhD Candidate
Department of Biology and Wildlife
University of Alaska-Fairbanks
Antibody performed very well
The tyrosine hydroxylase antibody was successfully used for immunostaining of dopamine neurons in the adult Drosophila brain. The antibody performed very well for this application.
Drosophila brains were collected and stained as follows:
1. Dissected in PBT buffer
2. Fixed in 4% PFA for 20 min at RT
3. Washed in PBT twice
4. Blocked in 5% normal goat serum in PBT buffer for 30 min at RT
5. Incubated in anti-TH primary (1:1000) for 48 h at 4 deg C
6. Washed 5 times in PBT
7. Incubated in anti-mouse alexa fluor-488 secondary antibody for 48h at 4 deg C
8. Washed 5 times in PBT before mounting and imaging
Dopamine neuron cell bodies and projections within the canonical anterior and posterior clusters could be readily visualized by confocal microscopy z-stack imaging. There was very little background staining in the Drosophila brain.
Submitted by:
Ian Martin, PhD
Jungers Center for Neurosciences Research
Parkinson Center of Oregon
Oregon Health and Science University
Consistent Results
I have used the mouse antibody for tyrosine hydroxylase (Product ID : 22941) for staining dopaminergic cells in Drosophila melanogaster adult brains, larval brains, as well as primary neuronal cultures, and have always had consistent results, as well as a good signal-to-noise ratio of 4 to 1.
In published results, I compared the staining of this antibody with a GFP signal driven by tyrosine hydroxylase and the overlap between the two signals was good (J Neurochem. 2013 Aug;126(4):529-40. doi: 10.1111/jnc.12228. Epub 2013 Mar 24).
Also, in unpublished results, it appeared to perform just as well as a Drosophila antibody for tyrosine hydroxylase. I have used this antibody for years and have always obtained consistent results.
Lyle Wiemerslage, PhD
Biomedicinska Centrum (BMC)
Uppsala Universitet
Sweden
Produces distinct staining with very low background
We have used the ImmunoStar Inc. tyrosine hydroxylase antibody (22941) with great success, for identifying TH-IR cells in the striatum and midbrain of European starling (Sturnus vulgaris). We typically use it at a 1:3000 dilution, which produces distinct staining with very low background.
Tissue preparation:
Birds are transcardially perfused with 1X phosphate-buffered saline (PBS), followed by 4% paraformaldehyde. The brain is extracted and post-fixed in 4% paraformaldehyde overnight, then transferred to 30% sucrose for 2-3 days (until the brain sinks). Brains are sectioned at 40 µm on a sliding microtome equipped with a freezing stage, and collected into PBS.
Immunohistochemistry procedure:
Brain sections are transferred to 24-well plates. All solutions are made up in 1X PBS and are typically applied at a volume of 0.5 ml. The plates are gently agitated on a shaker during incubations.
Day 1:
• Rinse in PBS 3 times, for 10 min each
• Incubate in 3% hydrogen peroxide for 10 min
• Rinse in PBS 3 times, for 10 min each
• Incubate in blocking serum (from Vector ABC Elite kit) + 0.2% Triton X-100 for 30 min
• Incubate in primary antibody + 0.5% Triton X-100 overnight at 4°C
Day 2:
• Rinse in PBS 3 times, for 10 min each
• Incubate in secondary antibody (from Vector ABC Elite kit) + 0.6% Triton X-100 for 30 min
• Rinse in PBS 3 times, for 10 min each
• Incubate in ABC reagent (from Vector ABC Elite kit) + 0.6% Triton X-100 for 30 min
• Rinse in PBS 3 times, for 10 min each
• Incubate in 0.05% DAB (3’3-diaminobenzidine) + 0.015% hydrogen peroxide for 2-3 min
• Rinse in PBS 3 times, for 10 min each
Float-mount sections onto gelatin-subbed slides and let dry overnight, then dehydrate with ethanol and xylene rinses and coverslip with Permount.
Joseph M. Casto, Ph.D.
Associate Professor
School of Biological Sciences
Campus Box 4120
Illinois State University
Normal, IL 61790-4120
Used this successfully for years
Tissue used: Free floating, 25-40µm thick sections from perfused mice (4% PFA followed by 10% sucrose dehydration).
Protocol steps: Standard IHC protocol using vector biolabs ABC mixture and DAB reagent
Concentration of TH antibody: 1:5000, left overnight in PBS+0.3% triton x-100
Staining results: Specific staining of typical dopaminergic neurons within the midbrain (VTA and SNc). Cytoplasmic staining.
I have used this successfully over the past years (see Rousseaux et al, 2012, PNAS; Aleyasin, Rousseaux et al, 2010, PNAS)
Submitted by:
Maxime Rousseaux, Ph.D.
Laboratory of Dr. Huda Y. Zoghbi
Department of Molecular and Human Genetics
Baylor College of Medicine
Perfection
We have gotten truly incredible results with the Immunostar TH antibody. We have been using the antibody to label tissue from several different invertebrates with fantastic results. The confocal images are un-real.
We fix tissue in 4% paraformaldehyde then rinse 3x (15min each) in PBS and place in a block solution overnight. After that we place it in a 1:100 dilution of this primary and leave it alone in the fridge for 4 days. Tissue is then placed in a dilutant solution and in secondary antibody in the fridge at least overnight. We then rinse, dehydrate, and mount.
We have used this protocol for moths, leeches, and spiders without issue.
Cynthia Harley
Department of Entomology
University of Minnesota
Works Consistently Great on Rat TH
Used it at 1:5000 overnight for immunofluorescence experiment (floating 40um sections) on rat locus coeruleus, and got stunning images. Also worked well in a colocalization experiment when mixed with a primary for glucocorticoid receptor (raised on rabbit).
Lishay Goozy Alaluf
Dept of Biochemistry and Molecular Biology
New York Medical College
Works Great!
I use this antibody regularly to stain for tyrosine hydroxylase in sections of rat retina. I have always been very happy with the results and found that the best working dilution to be 1:1000. It produces a strong image with very little background and I would definitely recommend this antibody to other users.
1. Fixed the tissue in 4% paraformaldehyde and PBS
2. Collected the eye and then sectioned it into 20 micron slices using a cryostat
3. Placed sections in blocking buffer for 1 hour
4. Placed the sections in primaries overnight:
1:5000 dilution of in house anti rat melanopsin
1:1000 dilution of your TH antibody
5. Put the sections in secondary antibodies for 2 hours:
1:2000 alexa fluor 488 dk anti rabbit
1:2000 alexa fluor 594 dk anti mouse
6. Then we imaged it using a max projection of a Zeiss laser scanning confocal.
Emily Gray
Dr. Lane Brown Lab
Head Laboratory Technician
Department of Physiology & Neuroscience
Washington State University
Wonderful Results
We have been using the Immunostar anti-tyrosine hydroxylase antibody on Drosophila brain tissue with wonderful results. No background labeling, very bright signal, and worked the very first time with no modifications to our protocol required! We made aliquots of the antibody as per the instructions (dilute 1:10 in PBS for storage in the freezer, then dilute an additional 1:5 for staining) and used the following protocol.
1. dissect brains in saline and fix for 20 minutes in 4% PFA
2. rinse 3x in PBST and place in block solution (5% NGS in PBST) for 20 minutes
3. place in primary antibody (1:50 total dilution in PBST) for 2 days at 4 degrees
4. rinse 3x in PBST
5. place in secondary antibody (1:400) for 2 days at 4 degrees
6. rinse 3x in PBST, then mount tissue on slides
Katherine A Tschida, Postdoctoral Associate
Duke University
Duke Biology
Durham, NC
A Fabulous Antibody!
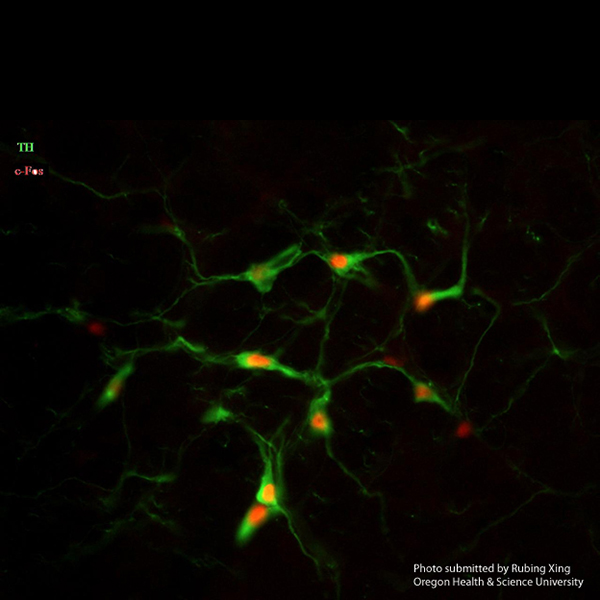
I have routinely used this monoclonal antibody to stain for tyrosine hydroxylase (#22941) in both rat and mouse brains for several years with consistent and excellent results. It is the best antibody out there! Over more than 10 years I have used different lots with equally high quality results. The best part of using this particular antibody is that staining is fabulously strong with high dilution rates up to 20,000x, so one batch can go a very long way. I even sometimes re-use a solution with good results still. Moreover, crisp soma dendrite and axonal staining can be achieved with both immunoperoxidase (using avidin-biotin complex) and immunofluorescence methods. It works great in multi-labeling studies in which I combined this antibody with immunolabeling of c-Fos or other markers.
Section preparation:
Mice and rats are perfused with 4% paraformaldehyde in 0.1M phosphate buffer (PB). Generally I add 15% of saturated picric acid solution, but for this staining it is not required. It may be informative to know that adding up to 0.5% glutaraldehyde does not affect the staining results (thus suitable for EM studies). After each brain is removed, it is post-fixed in 4% paraformaldehyde overnight, then blocked and sliced in 40 or 50 micrometer sections on a vibratome. Sections are collected free-floating in series in multi well plates and stored in 0.1M PB to which 0.1% sodium azide is added for long-term storage at +4°C.
Day 1.
The sections chosen for staining are then pretreated in 0.1% sodium borohydrate (0.1%) in PBS for 10 minutes, and also in 0.3% hydrogen peroxide 0.1% sodium azide mixture in PBS for 30 minutes to quench peroxidase activity. To suppress nonspecific binding sections are left overnight in a mixture of normal serum and Fab fragment of anti mouse IgG (usually goat serum and IgG – if use of goat secondary antibodies are appropriate). All antibody solutions are made up in PBS to which is added 0.1% sodium azide (for repeated uses) and 0.5% Triton X-100 (labeled PBS-T/A).
Days 2-3.
After 3 washes in PBS, sections are incubated for at least 24 h (often 2 days at 4 °C) in anti TH at a dilution of 10,000x in PBS-T/A containing 2% normal goat serum (NGS).
Day 3-4
Following washes in PBS sections are transferred to the secondary antibody (e.g. goat anti-mouse IgG-Alexa488 for or biotinylated goat anti-mouse IgG) in PBS-T/A with 2% NGS. This incubation can last from 4 h (at RT) or overnight at 4°C. For immunofluorescence sections can be mounted and evaluated after this step.
Day 4-5 and on.
Biotinylated secondary antibody incubation is followed by PBS washes, and transfer into Avidin-Biotin complex solution (Vector Labs) diluted 1000 x in PBS-T (no azide added this time!). This incubation lasts from 4 h to overnight at 4°C. After that the sections are rinsed in PBS and Tris-HCl solutions (pH 7.6) and stained using diaminobenzidine. I prefer not to use straight H2O2 but add glucose oxidase (1 microliter per 5 ml) to the DAB mixture and infuse D-glucose (50 ul of 10% solution per 5 ml) into the wells to start the reaction slowly.
Ronald P. Gaykema, Ph.D.
Department of Pharmacology
University of Virginia Health System
Charlottesville, VA 22908
Highly Recommend This Antibody
We have used the Tyrosine Hydroxylase antibody #22941 for western blotting on mouse brain lysates. The antibody is specific and gives clean bands. It works both using the ECL and the LICOR Odyssey system using infrared dyes.
I therefore highly recommend this antibody.
Kristina Becanovic, PhD
Department Clinical Neuroscience
Karolinska Institutet
171 76 Stockholm
One of the Best Antibodies
We have used this antibody (22941) to detect endogenous levels of TH in mouse and human cells. For cryosections, it works better after antigen retrieval (sodium citrate). The dilution we use is 1:250, and overnight incubation with the primary at 4°C increases the signal quite a lot.
It works beautifully giving a clear strong signal, with low background. One of the best antibodies to detect TH that we have found.
Lottie Jansson Sjostrand
Karolinska Institutet
Stockholm Sweden
Highly recommend!
The TH antibody worked very well with no issues or background. I highly recommend this antibody to my colleagues.
Zebra finch brain tissue embedded in gelatin (from perfused birds)
30um sections stored in cryoprotectant
Rinse 6 X 5 min in PBS
Wash 15 min in 0.5% H2O2 in PBS
Block 1 hour in 5% normal donkey serum in PBS with 0.3% TritonX (PBST)
Incubate in TH antibody (1:1,000 dilution) in PBST for 2 days at 4°C.
Rinse 3 X 5 min in PBS.
Incubate 1 hour in secondary antibody.
Rinse 3 X 5 min. in PBS @ RT.
ABC elite kit (Vector)-1 hour
Rinse 3 X 5 min in PBS
DAB plus 0.0075% H2O2 for 7 minutes.
Rinse in PBS.
Mount onto slides, dehydrate and coverslip with DPX
Dr. Juli S. Wade
Dr. Yu-Ping Tang
Neuroscience Program
Michigan State University
Beautiful Labeling Images
We used ImmunoStar Tyrosine Hydroxylase Antibody, Product ID: 22941, to label rat brain C1 region catecholaminergic cells, and got very beautiful labeling images.The protocol we used:
Day1
Select tissue sections (frozen, 35 um);
Rinse sections in PBS for 2×10 min on shaker at room temperature;
Incubate sections in ADS (Antibody dilution solution) for 2 hrs on shaker at room temperature;
Prepare primary antibody solution;
Ms anti-TH (ImmunoStar, Catalog: 22941), use at 1:1500;
Incubate sections in primary antibody solution overnight on shaker at room temperature;
Day2
Rinse sections in T-PBS for 2×10 min on shaker at room temperature;
Prepare secondary antibody solution;
Donkey anti Ms A488 (Molecular Probes, A21202), use at 1:500;
Incubate sections in secondary antibody solution for 1 hr on shaker at room temperature;
Rinse sections in PBS for 2×10 min on shaker at room temperature.
Mount sections on Superfrost Plus Microscope Slide.
Coverslip mounted sections with anti-fade mounting regent.
Rubing Xing, Senior Research Assistant
Neurological Surgery
Oregon Health & Science University
Exceptional Results
I have used your TH monoclonal antibody 22941 for many years with beautiful results. This is one of the antisera we use on an almost daily basis. We are extremely glad that you were able to confirm the specificity of the TH antibody in transfected cells.
Thank you and all the technical staff for the valuable assistance and your wonderful help in its characterization.
Review submitted by:
Virginia M. Pickel, PhD
Division of Neurobiology
Weill Cornell Medical College,